Happy Lunar New Year from the USC US-China Institute!
Jade, or not Jade? That is the Question
A collection of Chinese Jade Art is exhibited in Jade Room in Dallas Museum of Art.
Where
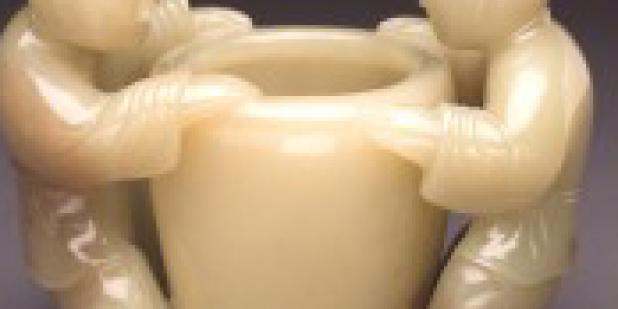
Jade is a word applied to many different stones–so many that it is useful now and then to re-claim mastery of the differences for understanding and appreciating Chinese “jade.” This exhibition draws on the Museum’s Collection and generous loans of natural worked stones to encourage just that. Works of art to which the term “jade” has been applied and misapplied are grouped by their discernable distinctions as stones, rather than by style, subject, function, or date they were worked. An exercise in training the eye is presented for the pleasure it affords, along with any practical benefits that may accrue for the collector. The value added of skillfull carving, pleasing shape, fine polish.
The exhibition is dominated by natural and worked stones most commonly prized among collectors of Chinese “Jade”: Nephrite and Jadeite—two distinct materials. Other stones that sometimes pass for “Jade” also appear to serve for visual comparison: Serpentine, Chrysoprase, Agate, Soapstone, Malachite, Opaque Dolomite Marble, and others that abound particularly in snuff-bottles for the delight of the discerning eye.
Nephrite and Jadeite are both legally traded as “Jade” in the international marketplace. Both are geologically identified as metamorphic rocks, materials transformed by heat, pressure, and water from igneous magma flowing into the earth’s mantle. Both are chemically classed among minerals as silicates, that is they are rich in silicon, the second most abundant element in the earth’s outer layer, but they are grouped differently because of dissimilar chemical compositions and physical structures. Among minerals, Nephrite is an amphibole, and Jadeite a pyroxene. Nephrite is not even technically a full-blooded mineral; it lacks a developed physical crystalline structure in which atoms bond into regular, repeating, three-dimensional shapes. The conditions of heat and pressure that transformed igneous rock to metamorphic Nephrite were followed by a phase of rapid cooling that inhibited the growth of crystals. It’s structure is described by minerologists as “crypto- or micro-crystalline.” The microscopic crystal structure of Nephrite is an irreglar felt-like bond of matted chains. Jadeite has a clearly defined crystal structure of octa-hedrally co-ordinated aluminum atoms and 8 co-ordinated sodium polyhedra connected by silicate chains running parallel to the C axis of the geometric form. The grainy structure of Jadeite is denser and harder than the fibrous arrangement of Nephrite: Jadeite is the more difficult, for example to scratch. The interwoven structure of Nephrite is more resistant to breakage, and by definition the tougher of the two compounds.
Featured Articles
We note the passing of many prominent individuals who played some role in U.S.-China affairs, whether in politics, economics or in helping people in one place understand the other.
Events
Ying Zhu looks at new developments for Chinese and global streaming services.
David Zweig examines China's talent recruitment efforts, particularly towards those scientists and engineers who left China for further study. U.S. universities, labs and companies have long brought in talent from China. Are such people still welcome?






